I consider myself as an educator. Someone who teaches others, thereby bestowing knowledge upon them. For as long as I can remember, I always enjoyed explaining things to others. The biggest joy I get from the experience is the feeling that the more I explain things, the more I understand them.
You see, when we sit down and learn something new, we usually start with remembering it. This can be done through repetition. If I sing the A,B,C enough times, I am sure to remember them. The problem is that just because I can remember something, doesn't mean I understand it. To understand, I need to be able to apply what it is I can remember. But even applying the knowledge can sometimes amount to nothing more than a technical skill.
However, when you try to explain it to someone else, that's when your knowledge is really put to the test. When you are being asked "why is it like this?" or "why can't you do it another way?" is when you have to critically examine the extent of your understanding.
Science can be hard to understand. Like any field of knowledge, science can be understood in many levels, and the higher you go, the more complicated it becomes. And the more complicated it becomes, the more assumptions you make, or take things as is, to allow you to understand the even more complex ideas.
Confused?
Here's an example.
We now know today that all matter is made up of atoms.
But what is an atom?
Is is a particle made up of smaller particles. The nucleus, positively charged, and electrons around it, negatively charged.
The nucleus is actually made up of protons, positively charged, and (except for Hydrogen) neutrons, without any charge.
We can go on and on, dissecting the particles even further, BUT, even before scientists discovered the existence of the electrons, the concept of atoms was still being used to explain what is matter. It was just assumed that there is this basic element called "atom" which has so and so characteristics, and with this assumption knowledge was formulated. Once electrons and protons were discovered, they became the fundamental elements used to explain, and so on and so forth.
In today's complex scientific world, the depth of knowledge is so great that scientists sometime forget that there's a huge canyon between the general public and the specialists. Which is why I find "The Flame Challenge" to be such a great effort to bring science back to the ground.
In a nutshell, the idea is this - you think you understand something really well? now try to put it in simple language so even an 11 year old can understand.
The first challenge was to explain what flame is. The second, what is time. The current challenge - what is color.
The challenge is not an easy one, because as we become more and more informed, more and more specialized, more and more knowledgeable, we tend to take a lot of concepts for granted, forgetting that most people either don't understand them or don't even know about them. We take our point of view as the obvious one, which may or may not be what other people might think.
Let's take color as an example. Without going into what color is, lets explore the various ways we can think of color.
Color is something we see. Objects appear in different colors. We may explore the property of matter, and why different things have different color.
Color is something we experience. We can explore how we perceive color. How does our eye register color and how does our brain interpret the signal coming from the eye.
Color is a form of energy. We can explore the meaning of color from a pure physical point of view.
There are probably more ways to think about color, and I didn't even start to explain any of them.
So what do you think? can you explain what is color to an 11 year old?
Monday, 16 December 2013
Saturday, 14 December 2013
Damme, I broke the laws of Physics again!
Are you one of the 60 millions (!!!) of people who watched Volvo's "Epic Split" ad featuring Jean-Claude Van Damme?
If not, here it is, with its majestic feel:
It is a beautiful ad. Very elegant, extremely impressive performance, and above all, amazing engineering by Volvo to allow such high precision steering of their trucks.
But, one thing it is not - defying the laws of physics.
I'm sorry to be a stickler for details, but with all due respect to Jean-Claude Van Damme (and much respect I do have for his mastering of the body and mind), physics is not the least surprised by this ad.
Which "laws of physics" did the screenwriters think of when they wrote this line?
Were they thinking of Newton's law of universal gravitation?
Are we to expect that Jean-Claude Van Damme should fall to the ground due to gravity, and surprisingly he does not? of course he does not fall, his legs are resting on the trucks' side mirrors.
Were they thinking of friction ?
Were we expecting Jean-Claude Van Damme's feet to slide off the side mirrors? obviously, he chose proper shoes which provide enough friction, as well as being able to control his balance such that he doesn't looses his foot hold.
What other 'Laws of Physics' could they have been thinking of?
Under "Major Laws of Physics" we can find a few others:
- E = m c2
(clearly Jean-Claude Van Damme does not transforms into energy)
- Conservation of momentum
(not colliding into anything, and with the trucks not breaking, momentum doesn't change anyways)
- Laws of thermodynamics
(its hard to say what is happening to Jean-Claude Van Damme's internal energy or his entropy, so I cannot comment on these ones)
- Electrostatic laws
(having no wires connected to him, nor a light bulb, I don't think Jean-Claude Van Damme is generating an electrostatic field)
- Theory of relativity
(the trucks are traveling at a speed far slower than the speed of light, so this can't be the right one)
- Quantum mechanics
(with a body mass of a human being, Quantum mechanics are just as accurate as classical mechanics)
So, to sum things up, do I think this is an amazing ad? YES!
Would I have thought it was an amazing ad without having the "defy the laws of physics" in it? YES!
Would you have felt any different of the ad if 'laws of physics' were not "defied"? I'm guessing no.
(Dear commercial copywriters, I know you are being paid to deliver super drama. But seriously, if the theme is not science fiction, don't insult people by stating over dramatizing empty claims.)
If not, here it is, with its majestic feel:
It is a beautiful ad. Very elegant, extremely impressive performance, and above all, amazing engineering by Volvo to allow such high precision steering of their trucks.
But, one thing it is not - defying the laws of physics.
I'm sorry to be a stickler for details, but with all due respect to Jean-Claude Van Damme (and much respect I do have for his mastering of the body and mind), physics is not the least surprised by this ad.
Which "laws of physics" did the screenwriters think of when they wrote this line?
Were they thinking of Newton's law of universal gravitation?
Are we to expect that Jean-Claude Van Damme should fall to the ground due to gravity, and surprisingly he does not? of course he does not fall, his legs are resting on the trucks' side mirrors.
Were they thinking of friction ?
Were we expecting Jean-Claude Van Damme's feet to slide off the side mirrors? obviously, he chose proper shoes which provide enough friction, as well as being able to control his balance such that he doesn't looses his foot hold.
What other 'Laws of Physics' could they have been thinking of?
Under "Major Laws of Physics" we can find a few others:
- E = m c2
(clearly Jean-Claude Van Damme does not transforms into energy)
- Conservation of momentum
(not colliding into anything, and with the trucks not breaking, momentum doesn't change anyways)
- Laws of thermodynamics
(its hard to say what is happening to Jean-Claude Van Damme's internal energy or his entropy, so I cannot comment on these ones)
- Electrostatic laws
(having no wires connected to him, nor a light bulb, I don't think Jean-Claude Van Damme is generating an electrostatic field)
- Theory of relativity
(the trucks are traveling at a speed far slower than the speed of light, so this can't be the right one)
- Quantum mechanics
(with a body mass of a human being, Quantum mechanics are just as accurate as classical mechanics)
So, to sum things up, do I think this is an amazing ad? YES!
Would I have thought it was an amazing ad without having the "defy the laws of physics" in it? YES!
Would you have felt any different of the ad if 'laws of physics' were not "defied"? I'm guessing no.
(Dear commercial copywriters, I know you are being paid to deliver super drama. But seriously, if the theme is not science fiction, don't insult people by stating over dramatizing empty claims.)
Tuesday, 3 December 2013
My Brush with Art
A lot of universities have a Faculty called "Arts and Science".
If the faculty segments its disciplines into "Arts" and "Science" does that imply that arts and sciences don't mix? are they mutually exclusive? or is there some overlap?
Such fundamental questions as "what makes something Art?" or "Can Science be considered as Art?" came to my mind as I was organizing a public event for Pueblo Science which was to be part of Culture Days weekend, held on September 28th 2013 weekend across Canada. I named the activity : "Painting with Science".
Luckily for me, I happen to have a friend who is an art curator, and who is better qualified to help me in my quest to understand where Art and Science meet, or whether they don't, than an art curator?
The short answer I got was: "it is art if you say so."
Well that's easy then, I just say my science demonstration is art, therefor it must be art.
But will people believe me just because I said so?
So I kept on questioning, "but will people believe me? why should they?"
And here lies the profound boundary (at least based on my interpretation):
In other words, art presents you with something to explore with your emotions, to think about how you feel about it, to like it or dislike it, or perhaps to be indifferent, and in either way, ponder about why is it that you feel the way you feel, and then perhaps change your mind, feel something else. You may feel differently every time you experience it. And every person may feel differently about the same art.
Science, on the other hand, is about understanding why things are the way they are, why things behave the way they do. Your emotions are not part of it. Like it or not, gravity will pull you down when you loose your balance. Love it or not, but a drop of food colouring falling on a piece of fabric will soak and spread. Science is about articulating an explanation (and later testing the boundaries and limitations of that explanation). Finding a 'general rule' which will allow you to predict how the world will behave based on how it was observed to behave until now. And it doesn't matter who is the observer, the science is always the same.
Wait, so does that mean art and science can or cannot mix?
My feelings about this is that they can overlap if you let them.
If you ALLOW yourself to both FEEL as well as REASON, you can enjoy both ART and SCIENCE.
Look at this painting we had both adults and children paint at the event:
You can clearly see a canvas. You can also see different drawings, using different colors, pink and green. You can think about how the drawings make you feel. What do they remind you of. How the collection of different drawings produced by different people combine or clash. It is art produced by random people who were presented with a fabric, paint brushes and paint, and the opportunity to draw anything they felt like on a nice sunny morning in Toronto.
Oh, and one last thing you can't see from the image. The paint they were given were all colorless, transparent liquids!!! Yes, that's right. Our painters used solutions which looked exactly the same, but produced different colors when they touched the fabric. What a surprise. Now another feeling comes into play, that of surprise. Amazement.
And now, once you've let yourself soak the feeling of marvel, you may ask "Why does this happen?"
And we can now search for an explanation. Are the liquids the same or are they different? Is it the paint, the fabric, or their combination which produces the different colors?
We've stepped from an artistic experience into a scientific experience.
And once we understand why things happen, does that diminish our feelings? or maybe it enhances them? personally, I prefer to think of it in a non-competitive way. Our feelings are different with the knowledge we gained, but feelings are still feelings, no right ones no wrong ones. No better, no worse.
Think of how your feelings change when you learn how a magic trick was performed. The first time you see it, you are amazed. Once you learn how it was done, you may feel admiration towards the magician who has mastered the trick so well. Instead of replacing one feeling for another, cherish them both. Both add up to make you who you are.
So, as I explore my personal feelings about the who event, I conclude that I've learned that I can appreciate the artistic merit of my science activity. And I liked it.
If the faculty segments its disciplines into "Arts" and "Science" does that imply that arts and sciences don't mix? are they mutually exclusive? or is there some overlap?
Such fundamental questions as "what makes something Art?" or "Can Science be considered as Art?" came to my mind as I was organizing a public event for Pueblo Science which was to be part of Culture Days weekend, held on September 28th 2013 weekend across Canada. I named the activity : "Painting with Science".
Luckily for me, I happen to have a friend who is an art curator, and who is better qualified to help me in my quest to understand where Art and Science meet, or whether they don't, than an art curator?
The short answer I got was: "it is art if you say so."
Well that's easy then, I just say my science demonstration is art, therefor it must be art.
But will people believe me just because I said so?
So I kept on questioning, "but will people believe me? why should they?"
And here lies the profound boundary (at least based on my interpretation):
ART provokes your subjective FEELINGS
SCIENCE provokes your objective REASONING
In other words, art presents you with something to explore with your emotions, to think about how you feel about it, to like it or dislike it, or perhaps to be indifferent, and in either way, ponder about why is it that you feel the way you feel, and then perhaps change your mind, feel something else. You may feel differently every time you experience it. And every person may feel differently about the same art.
Science, on the other hand, is about understanding why things are the way they are, why things behave the way they do. Your emotions are not part of it. Like it or not, gravity will pull you down when you loose your balance. Love it or not, but a drop of food colouring falling on a piece of fabric will soak and spread. Science is about articulating an explanation (and later testing the boundaries and limitations of that explanation). Finding a 'general rule' which will allow you to predict how the world will behave based on how it was observed to behave until now. And it doesn't matter who is the observer, the science is always the same.
Wait, so does that mean art and science can or cannot mix?
My feelings about this is that they can overlap if you let them.
If you ALLOW yourself to both FEEL as well as REASON, you can enjoy both ART and SCIENCE.
Look at this painting we had both adults and children paint at the event:
You can clearly see a canvas. You can also see different drawings, using different colors, pink and green. You can think about how the drawings make you feel. What do they remind you of. How the collection of different drawings produced by different people combine or clash. It is art produced by random people who were presented with a fabric, paint brushes and paint, and the opportunity to draw anything they felt like on a nice sunny morning in Toronto.
Oh, and one last thing you can't see from the image. The paint they were given were all colorless, transparent liquids!!! Yes, that's right. Our painters used solutions which looked exactly the same, but produced different colors when they touched the fabric. What a surprise. Now another feeling comes into play, that of surprise. Amazement.
And now, once you've let yourself soak the feeling of marvel, you may ask "Why does this happen?"
And we can now search for an explanation. Are the liquids the same or are they different? Is it the paint, the fabric, or their combination which produces the different colors?
We've stepped from an artistic experience into a scientific experience.
And once we understand why things happen, does that diminish our feelings? or maybe it enhances them? personally, I prefer to think of it in a non-competitive way. Our feelings are different with the knowledge we gained, but feelings are still feelings, no right ones no wrong ones. No better, no worse.
Think of how your feelings change when you learn how a magic trick was performed. The first time you see it, you are amazed. Once you learn how it was done, you may feel admiration towards the magician who has mastered the trick so well. Instead of replacing one feeling for another, cherish them both. Both add up to make you who you are.
So, as I explore my personal feelings about the who event, I conclude that I've learned that I can appreciate the artistic merit of my science activity. And I liked it.
Monday, 28 October 2013
Don't give up!
No, I haven't given up on blogging just yet.
No, I haven't forgotten either.
I'm just tied up trying hard to finish my thesis.
More to come, I promise.
No, I haven't forgotten either.
I'm just tied up trying hard to finish my thesis.
More to come, I promise.
Wednesday, 25 September 2013
The beholder has eyes, but so do I
You know the old saying "Beauty is in the eye of the beholder" ?
(just for reference, this source claims Margaret Wolfe Hungerford is credited with the earliest appearance in her book Molly Bawn, dated 1878).
Anyways, I am currently in the midst of organizing and promoting an event which will take place this coming Saturday in the Chemistry building at UofT.
The event is part of the national Culture Days weekend which is dedicated to "raise the awareness, accessibility, participation and engagement of Canadians in the arts and cultural life of their communities"
For me, Science is a big part of my culture. I rely on science to decide which food to buy (or not buy), which cleaning products to use (or not use), and so many other decisions we all make every single day, entangled in our habits, our ideas, our opinions, our passions, our perception.
But wait, there's more. Science is not just part of our culture. It is also an object (if one can classify science as an object) of BEAUTY.
I think SCIENCE is BEAUTIFUL.
This is my subjective perception. I am the beholder.
And so many others share this feeling too (here's a few of them):
We all have a different idea of what it is in science that we find beautiful.
It is the surprise, the challenges, the novelty?
Is it the final answer or the process of getting that answer?
There is no right answer. They are all equally correct. Isn't that beautiful in itself?
So what will we do to show people how beautiful science can be?
Well, thank you for asking. We will be making paintings. But not in the ordinary fashion. No. We will try to surprise you in the way we draw.
And we'll also show some neat chemistry which you can do to create art.
(can you tell I'm being careful not giving away all our secrets just yet... but I do promise to post pictures and videos after the event is over)
So what is it that I'm trying to say?
Well, that sometimes, it is nice to stop thinking about explaining science. Sometimes, its nice to think about how you feel about science.
(just for reference, this source claims Margaret Wolfe Hungerford is credited with the earliest appearance in her book Molly Bawn, dated 1878).
Anyways, I am currently in the midst of organizing and promoting an event which will take place this coming Saturday in the Chemistry building at UofT.
The event is part of the national Culture Days weekend which is dedicated to "raise the awareness, accessibility, participation and engagement of Canadians in the arts and cultural life of their communities"
For me, Science is a big part of my culture. I rely on science to decide which food to buy (or not buy), which cleaning products to use (or not use), and so many other decisions we all make every single day, entangled in our habits, our ideas, our opinions, our passions, our perception.
But wait, there's more. Science is not just part of our culture. It is also an object (if one can classify science as an object) of BEAUTY.
I think SCIENCE is BEAUTIFUL.
This is my subjective perception. I am the beholder.
And so many others share this feeling too (here's a few of them):
We all have a different idea of what it is in science that we find beautiful.
It is the surprise, the challenges, the novelty?
Is it the final answer or the process of getting that answer?
There is no right answer. They are all equally correct. Isn't that beautiful in itself?
So what will we do to show people how beautiful science can be?
Well, thank you for asking. We will be making paintings. But not in the ordinary fashion. No. We will try to surprise you in the way we draw.
And we'll also show some neat chemistry which you can do to create art.
(can you tell I'm being careful not giving away all our secrets just yet... but I do promise to post pictures and videos after the event is over)
So what is it that I'm trying to say?
Well, that sometimes, it is nice to stop thinking about explaining science. Sometimes, its nice to think about how you feel about science.
Thursday, 19 September 2013
For the Love of Science
Sometimes others can articulate your thoughts so much better than
you, at which point your best choice is to nod and say "you just read my
thoughts".
John Skylar put it beautifully on his website:
http://www.johnskylar.com/post/61507282912/why-you-dont-fucking-love-science
Thank you John.
John Skylar put it beautifully on his website:
http://www.johnskylar.com/post/61507282912/why-you-dont-fucking-love-science
Thank you John.
Saturday, 10 August 2013
A Table Tale
(The following piece was first posted on www.chemicalsareyourfriends.com, to which I am now contributing my thoughts on Chemistry. I will, however, keep on posting stuff which is not strictly chemistry here, in addition to mirroring my writing on Chemistry)
It is merely a table. Or is it.... I first heard Tom Lehrer sing "The Elements" song when I was taking my first year general chemistry course more than a decade ago.
The song lists all the elements known at the time, which was only 102, compared to the 114 officially recognized elements we have today, all sang to the tune of Gilbert and Sullivan's Major-General's song.
The final words of the song are:
And here lies the true wonder of the graphical masterpiece commonly known as "The Periodic Table of the Elements". Its name is misleading, since by the use of the word 'table' one may expect nothing more than "an orderly arrangement of data". But the periodic table is actually something completely different.
It is ... (wait for it, building the suspense here) .... A GRAPH!!!!
Or more precisely, an amalgamation of many graphs!!!
Yes, that's the truth.
What is the difference you may ask?
Well, a table is usually a way of presenting information in a tidy fashion to make it easier to find specific information of relevance. But a graph is a much more powerful tool. A graph plots values that are correlated to two or more attributes. Once plotted, trends can sometimes be observed. And if a trend exists - you can PREDICT! After all, science is more than just observing nature and taking notes. Science is about using the earlier observations in order to predict the outcome of future experiments (aka forming hypotheses)!
When Dimitri Ivanovich Mendeleev first published his periodic table of the elements in 1869, the elements were (for the most part) arranged based on their molecular weights. Mendeleev noticed that when arranging the elements according to their molecular weights (since atomic numbers were not yet a measurable quantity at that time - see footnote), you can arrange the elements in such a way that certain periodicities arise with respect to the properties of the elements. But the true breakthrough in Mendeleev's approach was that he then utilized his discovered pattern to predict new elements which had not yet been discovered. By using the periodic trends in the properties of the elements, he was able to predict some of the properties of those yet-to-be-discovered elements. And guess what ... he was right. Shortly after, Gallium, Scandium and Germanium were discovered, corroborating Mendeleev's hypothesis and exemplifying the practicality of the periodic table of the elements.
I mentioned the word "periodicity" several times, but periodicity of what? The answers is: quite a fair bit. Let's look at how the Ionization energy of the various elements changes when ordered in the periodic table arrangement. (The ionization energy is the amount of energy needed to separate one electron from the initially neutral atom). In the above graph, the height of each element corresponds to its first ionization energy (in eV units).
So what can we tell by looking at the graph? The first obvious observation is that when moving down each column, the height decreases (granted there are some exceptions, but let's look at the general rule). Another observation is that when moving from left to right along each row, the height generally increases, although several sharp drops are seen (such as in the case of N-nitrogen and O-oxygen or Cd-cadmium and In-indium).
You can check out some more examples at www.chemicool.com .
Atomic radius, ionization energy, melting point, boiling point, density; they all show a periodic behavior when plotted against their atomic numbers (again see footnote).
Why do we get such periodicities you might be wondering?
The atomic number, the number of protons in the nucleus of the element, provides the basis of the periodicities in the periodic table. The more protons there are , the heavier the atom is. Additionally, the atomic number also indicates the number of electrons around the nucleus (since the atoms in their pure state are neutral, therefore for every positively charged proton in the nucleus there will be a negatively charged electron around it). As the number of electrons increases, the atomic radius becomes larger (with exception of 'kinks' due to periodic changes in the arrangement of the electrons, similar to the sudden sharp drops we saw for the ionization energies). And since electrons are the main players in chemical reactions, the number of electrons and their specific arrangement around the nucleus will affect the reactivity of the element.
In chemistry, electrons like to be paired. Just like people (nudge, nudge, wink, wink). Let's take a look at the group with a common attribute along a vertical line in the periodic table: Lithium, Sodium and Potassium. They all have a single unpaired electron, and are similarly reactive because an unpaired electron is more reactive being all by itself.
In the above graph, the height of each element corresponds to its first ionization energy (in eV units).
So what can we tell by looking at the graph? The first obvious observation is that when moving down each column, the height decreases (granted there are some exceptions, but let's look at the general rule). Another observation is that when moving from left to right along each row, the height generally increases, although several sharp drops are seen (such as in the case of N-nitrogen and O-oxygen or Cd-cadmium and In-indium).
You can check out some more examples at www.chemicool.com .
Atomic radius, ionization energy, melting point, boiling point, density; they all show a periodic behavior when plotted against their atomic numbers (again see footnote).
Why do we get such periodicities you might be wondering?
The atomic number, the number of protons in the nucleus of the element, provides the basis of the periodicities in the periodic table. The more protons there are , the heavier the atom is. Additionally, the atomic number also indicates the number of electrons around the nucleus (since the atoms in their pure state are neutral, therefore for every positively charged proton in the nucleus there will be a negatively charged electron around it). As the number of electrons increases, the atomic radius becomes larger (with exception of 'kinks' due to periodic changes in the arrangement of the electrons, similar to the sudden sharp drops we saw for the ionization energies). And since electrons are the main players in chemical reactions, the number of electrons and their specific arrangement around the nucleus will affect the reactivity of the element.
In chemistry, electrons like to be paired. Just like people (nudge, nudge, wink, wink). Let's take a look at the group with a common attribute along a vertical line in the periodic table: Lithium, Sodium and Potassium. They all have a single unpaired electron, and are similarly reactive because an unpaired electron is more reactive being all by itself.
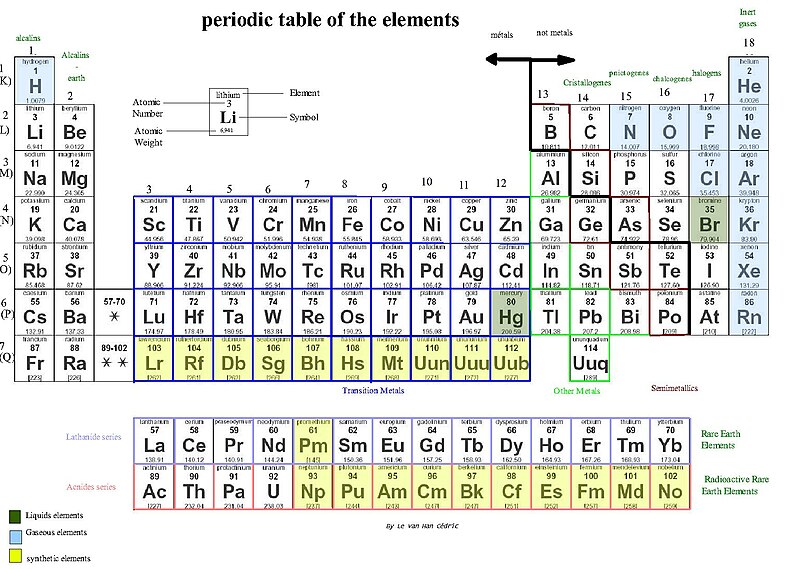 Contrary to the above group of elements, Helium, Neon and Argon have all their electrons paired. They are all similarly nonreactive and belong to the same group along a vertical line in the periodic table.
(In fact, the electronic structure is more complicated than simple pairing, which is why we saw fluctuations in the above graph where the ionization energy showed sharp drops in the general increasing trend moving from left to right along the rows. Since this post is getting quite long, I'll leave such descriptions to another time)
The periodic 'table' contains a plethora of information. Graphically, it is (probably) the most concise form to summarize an astounding amount of information.
Contrary to the above group of elements, Helium, Neon and Argon have all their electrons paired. They are all similarly nonreactive and belong to the same group along a vertical line in the periodic table.
(In fact, the electronic structure is more complicated than simple pairing, which is why we saw fluctuations in the above graph where the ionization energy showed sharp drops in the general increasing trend moving from left to right along the rows. Since this post is getting quite long, I'll leave such descriptions to another time)
The periodic 'table' contains a plethora of information. Graphically, it is (probably) the most concise form to summarize an astounding amount of information.
So next time you gaze at the periodic table, remember, it is more than 'just' a table. It is the essence of the chemistry that makes up our entire universe!
Footnote: The numbers we see today as the basic ordering of the elements are the Atomic Numbers. These are the number of protons in the nucleus of each of the elements, but they were only discovered in 1913 by Henry Moseley, which makes Mendeleev's accomplishment even more impressive.
It is merely a table. Or is it.... I first heard Tom Lehrer sing "The Elements" song when I was taking my first year general chemistry course more than a decade ago.
"These are the only ones of which the news has come to Harvard,
And there may be many others, but they haven't been discahvahd"
And here lies the true wonder of the graphical masterpiece commonly known as "The Periodic Table of the Elements". Its name is misleading, since by the use of the word 'table' one may expect nothing more than "an orderly arrangement of data". But the periodic table is actually something completely different.
It is ... (wait for it, building the suspense here) .... A GRAPH!!!!
Or more precisely, an amalgamation of many graphs!!!
Yes, that's the truth.
What is the difference you may ask?
Well, a table is usually a way of presenting information in a tidy fashion to make it easier to find specific information of relevance. But a graph is a much more powerful tool. A graph plots values that are correlated to two or more attributes. Once plotted, trends can sometimes be observed. And if a trend exists - you can PREDICT! After all, science is more than just observing nature and taking notes. Science is about using the earlier observations in order to predict the outcome of future experiments (aka forming hypotheses)!
When Dimitri Ivanovich Mendeleev first published his periodic table of the elements in 1869, the elements were (for the most part) arranged based on their molecular weights. Mendeleev noticed that when arranging the elements according to their molecular weights (since atomic numbers were not yet a measurable quantity at that time - see footnote), you can arrange the elements in such a way that certain periodicities arise with respect to the properties of the elements. But the true breakthrough in Mendeleev's approach was that he then utilized his discovered pattern to predict new elements which had not yet been discovered. By using the periodic trends in the properties of the elements, he was able to predict some of the properties of those yet-to-be-discovered elements. And guess what ... he was right. Shortly after, Gallium, Scandium and Germanium were discovered, corroborating Mendeleev's hypothesis and exemplifying the practicality of the periodic table of the elements.
I mentioned the word "periodicity" several times, but periodicity of what? The answers is: quite a fair bit. Let's look at how the Ionization energy of the various elements changes when ordered in the periodic table arrangement. (The ionization energy is the amount of energy needed to separate one electron from the initially neutral atom).
 In the above graph, the height of each element corresponds to its first ionization energy (in eV units).
So what can we tell by looking at the graph? The first obvious observation is that when moving down each column, the height decreases (granted there are some exceptions, but let's look at the general rule). Another observation is that when moving from left to right along each row, the height generally increases, although several sharp drops are seen (such as in the case of N-nitrogen and O-oxygen or Cd-cadmium and In-indium).
You can check out some more examples at www.chemicool.com .
Atomic radius, ionization energy, melting point, boiling point, density; they all show a periodic behavior when plotted against their atomic numbers (again see footnote).
Why do we get such periodicities you might be wondering?
The atomic number, the number of protons in the nucleus of the element, provides the basis of the periodicities in the periodic table. The more protons there are , the heavier the atom is. Additionally, the atomic number also indicates the number of electrons around the nucleus (since the atoms in their pure state are neutral, therefore for every positively charged proton in the nucleus there will be a negatively charged electron around it). As the number of electrons increases, the atomic radius becomes larger (with exception of 'kinks' due to periodic changes in the arrangement of the electrons, similar to the sudden sharp drops we saw for the ionization energies). And since electrons are the main players in chemical reactions, the number of electrons and their specific arrangement around the nucleus will affect the reactivity of the element.
In chemistry, electrons like to be paired. Just like people (nudge, nudge, wink, wink). Let's take a look at the group with a common attribute along a vertical line in the periodic table: Lithium, Sodium and Potassium. They all have a single unpaired electron, and are similarly reactive because an unpaired electron is more reactive being all by itself.
In the above graph, the height of each element corresponds to its first ionization energy (in eV units).
So what can we tell by looking at the graph? The first obvious observation is that when moving down each column, the height decreases (granted there are some exceptions, but let's look at the general rule). Another observation is that when moving from left to right along each row, the height generally increases, although several sharp drops are seen (such as in the case of N-nitrogen and O-oxygen or Cd-cadmium and In-indium).
You can check out some more examples at www.chemicool.com .
Atomic radius, ionization energy, melting point, boiling point, density; they all show a periodic behavior when plotted against their atomic numbers (again see footnote).
Why do we get such periodicities you might be wondering?
The atomic number, the number of protons in the nucleus of the element, provides the basis of the periodicities in the periodic table. The more protons there are , the heavier the atom is. Additionally, the atomic number also indicates the number of electrons around the nucleus (since the atoms in their pure state are neutral, therefore for every positively charged proton in the nucleus there will be a negatively charged electron around it). As the number of electrons increases, the atomic radius becomes larger (with exception of 'kinks' due to periodic changes in the arrangement of the electrons, similar to the sudden sharp drops we saw for the ionization energies). And since electrons are the main players in chemical reactions, the number of electrons and their specific arrangement around the nucleus will affect the reactivity of the element.
In chemistry, electrons like to be paired. Just like people (nudge, nudge, wink, wink). Let's take a look at the group with a common attribute along a vertical line in the periodic table: Lithium, Sodium and Potassium. They all have a single unpaired electron, and are similarly reactive because an unpaired electron is more reactive being all by itself. Contrary to the above group of elements, Helium, Neon and Argon have all their electrons paired. They are all similarly nonreactive and belong to the same group along a vertical line in the periodic table.
(In fact, the electronic structure is more complicated than simple pairing, which is why we saw fluctuations in the above graph where the ionization energy showed sharp drops in the general increasing trend moving from left to right along the rows. Since this post is getting quite long, I'll leave such descriptions to another time)
The periodic 'table' contains a plethora of information. Graphically, it is (probably) the most concise form to summarize an astounding amount of information.
Contrary to the above group of elements, Helium, Neon and Argon have all their electrons paired. They are all similarly nonreactive and belong to the same group along a vertical line in the periodic table.
(In fact, the electronic structure is more complicated than simple pairing, which is why we saw fluctuations in the above graph where the ionization energy showed sharp drops in the general increasing trend moving from left to right along the rows. Since this post is getting quite long, I'll leave such descriptions to another time)
The periodic 'table' contains a plethora of information. Graphically, it is (probably) the most concise form to summarize an astounding amount of information.So next time you gaze at the periodic table, remember, it is more than 'just' a table. It is the essence of the chemistry that makes up our entire universe!
Footnote: The numbers we see today as the basic ordering of the elements are the Atomic Numbers. These are the number of protons in the nucleus of each of the elements, but they were only discovered in 1913 by Henry Moseley, which makes Mendeleev's accomplishment even more impressive.
Tuesday, 16 July 2013
Science Fair at the Science Camp
Wow, it is sooooo hot and humid these days in Toronto.
And like every summer, Toronto is bustling with tourists.
But more than that, Toronto is a popular place for people from all over the world to come and improve their English skills, while soaking up sun and sites of this gorgeous place.
How does that have anything to do with science?
Well, as it happens, this last Saturday, as part of the Pueblo Science experience, we held a "Science Fair" event for the CISS ESL camp at St. Michael's University.
We had a GREAT time!!!!
We had:
Balloons pushed into Liquid Nitrogen
What you see:
When an inflated balloon is pushed into liquid nitrogen it shrinks.
When taking it out of the liquid nitrogen, it expands back to its former size.
Why does that happen?
That's because the trapped air inside shrinks when the temperatures drop, and expands when the temperature increases.
The pressure inside is always constant at 1atm, same as in the atmosphere.
The amount of air molecules which are mainly nitrogen molecules and oxygen molecules is kept fixed because the balloon is closed tight.
The only two variables left to be changed are the temperature (liquid nitrogen is at -196 centigrade!) and the volume.
The relation between all these attributes (pressure, volume, amount and temperature) is called:
The Ideal Gas Law, which is P*V = n*R*T
(P is the pressure,
V is the volume
n is the amount of molecules
T is the temperature
and R is a constant that relates all of the above to one another and is called not surprisingly - the gas constant)
And also:
Non-Newtonian Fluid
What you see:
When you mix corn starch and water, you get a gooey mixture (very slimy).
When you push your finger in it slowly, it goes all the way in.
When you pound it hard and fast, it cannot penetrate. The mixture appears 'solid'.
Why does that happen?
Newton was able to formulate how fluids behave by stating that when you apply a force on them, the fluid will flow. But like all motion, fluids experience "friction" which is referred to as viscosity.
What Newton saw was that viscosity was constant of the material, and only changed with temperature.
However, some fluids (like our corn starch) behave differently. (therefore the term - "non-Newtonian")
Our stuff 'solidifies' with strong force, meaning its viscosity increases with force.
When the force is weak, the viscosity is lower.
There were a few more activities, but I think this post is long enough.
I'll continue this another time.
Stay tuned....
And like every summer, Toronto is bustling with tourists.
But more than that, Toronto is a popular place for people from all over the world to come and improve their English skills, while soaking up sun and sites of this gorgeous place.
How does that have anything to do with science?
Well, as it happens, this last Saturday, as part of the Pueblo Science experience, we held a "Science Fair" event for the CISS ESL camp at St. Michael's University.
We had a GREAT time!!!!
We had:
Balloons pushed into Liquid Nitrogen
What you see:
When an inflated balloon is pushed into liquid nitrogen it shrinks.
When taking it out of the liquid nitrogen, it expands back to its former size.
Why does that happen?
That's because the trapped air inside shrinks when the temperatures drop, and expands when the temperature increases.
The pressure inside is always constant at 1atm, same as in the atmosphere.
The amount of air molecules which are mainly nitrogen molecules and oxygen molecules is kept fixed because the balloon is closed tight.
The only two variables left to be changed are the temperature (liquid nitrogen is at -196 centigrade!) and the volume.
The relation between all these attributes (pressure, volume, amount and temperature) is called:
The Ideal Gas Law, which is P*V = n*R*T
(P is the pressure,
V is the volume
n is the amount of molecules
T is the temperature
and R is a constant that relates all of the above to one another and is called not surprisingly - the gas constant)
We also had:
The Disappearing Vial
What you see:
When you submerge a glass vial into a glass filled with oil, the vial becomes invisible!!!!!!
Why does this happen?
The reason we can see things is because light hits them, bounces back, and hits our eyes.
But the medium around the object also plays a role.
For instance, we look at a coin on the table, it is easy to see it and grab it.
But when the coin is in a pool of water, we see it, but have a herder time grabbing it.
This is because the water bends the light as it penetrates it. This bending of light is called REFRACTION, and the extent by which light is refracted is called "Refractive Index"
When light passed from one medium (say water) to another medium (say glass) the light will bend if the refractive indexes are not the same.
BUT, if the refractive indexes are the same (like in our case with oil and glass), then light passes through without bending (or, refracting) going straight through. This makes the glass appear invisible!
Non-Newtonian Fluid
What you see:
When you mix corn starch and water, you get a gooey mixture (very slimy).
When you push your finger in it slowly, it goes all the way in.
When you pound it hard and fast, it cannot penetrate. The mixture appears 'solid'.
Why does that happen?
Newton was able to formulate how fluids behave by stating that when you apply a force on them, the fluid will flow. But like all motion, fluids experience "friction" which is referred to as viscosity.
What Newton saw was that viscosity was constant of the material, and only changed with temperature.
However, some fluids (like our corn starch) behave differently. (therefore the term - "non-Newtonian")
Our stuff 'solidifies' with strong force, meaning its viscosity increases with force.
When the force is weak, the viscosity is lower.
There were a few more activities, but I think this post is long enough.
I'll continue this another time.
Stay tuned....
Friday, 5 July 2013
Misleading titles - real science, false impression
Science breakthroughs are exciting. They change our lives, they hold the promise for a better world.
Sometimes the explanation is straight forward, and can be easily understood by most people, even if they don't have any related background.
But sometimes, in the process of trying to convey breakthrough research to the ordinary person, editors (or bloggers, or twitters, or facebookers or .....) pick up on a concept they know about (even partially) and use that as the "catch", the title that will make people want to read the article/watch the video.
The problem?
Creating a false notion in people's perception. Misleading them to think something which is (scientifically speaking) is not true.
Today's example (and there are examples like this one popping out too often than one would like to admit):
"Doctors Take A Long Shot And Inject HIV Into Dying Girl. The Reason Why Will Amaze You."
But the real science is more subtle than that, as carefully outlined by Cancer Research UK:
http://scienceblog.cancerresearchuk.org/2013/06/25/no-doctors-did-not-inject-hiv-into-a-dying-girl-to-treat-her-cancer/
Their most important message to the public is:
"To be absolutely clear, the doctors in the video did NOT inject HIV – nor a “deadly disease” – into a child."
The reason for the misleading title is pinned to the fact that:
"According to the video ... the virus used in these experiments was originally derived from HIV, ... However, the virus has undergone significant genetic tinkering, meaning that it is no longer harmful ... And it’s arguable whether it should even be referred to as HIV at all, given how much it has been altered."
What really happened was that the HIV was used to alter the patient's own immune cells, to allow them to "infect" the rest of the body's immune cells with a new genetic trait (the one that kills the cancer cells).
Perhaps one can be forgiving, saying "but you admit that they used HIV, so what's all the fuss?"
The problem is that with such a title, people get the impression that HIV was the cure, where is fact, it was simply a "tool" to reprogram the body's immune cells.
Would you believe me if I told you I painted my house with Acetone? you would think this is odd.
But if I used Acetone as a paint thinner, and painted my house with the "modified" paint, you would naturally say that claiming I painted my house with Acetone is misleading. Yes, Acetone was part of the paint, but saying I painted with Acetone gives you the wrong impression.
Exactly like the story of the HIV and cancer cure.
Words are powerful.
Use them wisely.
Sometimes the explanation is straight forward, and can be easily understood by most people, even if they don't have any related background.
But sometimes, in the process of trying to convey breakthrough research to the ordinary person, editors (or bloggers, or twitters, or facebookers or .....) pick up on a concept they know about (even partially) and use that as the "catch", the title that will make people want to read the article/watch the video.
The problem?
Creating a false notion in people's perception. Misleading them to think something which is (scientifically speaking) is not true.
Today's example (and there are examples like this one popping out too often than one would like to admit):
"Doctors Take A Long Shot And Inject HIV Into Dying Girl. The Reason Why Will Amaze You."
But the real science is more subtle than that, as carefully outlined by Cancer Research UK:
http://scienceblog.cancerresearchuk.org/2013/06/25/no-doctors-did-not-inject-hiv-into-a-dying-girl-to-treat-her-cancer/
Their most important message to the public is:
"To be absolutely clear, the doctors in the video did NOT inject HIV – nor a “deadly disease” – into a child."
The reason for the misleading title is pinned to the fact that:
"According to the video ... the virus used in these experiments was originally derived from HIV, ... However, the virus has undergone significant genetic tinkering, meaning that it is no longer harmful ... And it’s arguable whether it should even be referred to as HIV at all, given how much it has been altered."
What really happened was that the HIV was used to alter the patient's own immune cells, to allow them to "infect" the rest of the body's immune cells with a new genetic trait (the one that kills the cancer cells).
Perhaps one can be forgiving, saying "but you admit that they used HIV, so what's all the fuss?"
The problem is that with such a title, people get the impression that HIV was the cure, where is fact, it was simply a "tool" to reprogram the body's immune cells.
Would you believe me if I told you I painted my house with Acetone? you would think this is odd.
But if I used Acetone as a paint thinner, and painted my house with the "modified" paint, you would naturally say that claiming I painted my house with Acetone is misleading. Yes, Acetone was part of the paint, but saying I painted with Acetone gives you the wrong impression.
Exactly like the story of the HIV and cancer cure.
Words are powerful.
Use them wisely.
Thursday, 27 June 2013
Distillations
I just received the University of Toronto CHEMISTRY ALUMNI MAGAZINE called DISTILLATIONS.
I was honored to be included in the "Graduate Profiles" for this addition
as well as mentioning the work Pueblo Science does, promoting science literacy
I want to take the opportunity to personally thank Penny and Nina from the Chemistry Department for their constant support, and for the wonderful job they are doing.
Thursday, 20 June 2013
Pool party gone scientifically wrong
Can liquid nitrogen react with the hypochloric acid in the swimming pool (which is the chemical form in which we use chlorine in pool water)?
Some people seems to think so.... and newspapers seem to publish this nonsense:
http://www.news.com.au/world-news/liquid-nitrogen-stunt-at-jagermeister-party-in-mexico-leaves-a-man-in-coma/story-fndir2ev-1226666959021
Lets get the fact right this time:
Nitrogen, in our case is the molecular form of nitrogen N-N or N2 (as opposed to the Nitrogen atom) is found in our atmosphere as gas, and accounts for about 78% (source: http://www.space.com/17683-earth-atmosphere.html). Thanks to technological innovations we can now make Liquid Nitrogen (http://blogs.howstuffworks.com/2009/07/27/how-do-they-make-liquid-nitrogen/)/
The boiling temperature of liquid nitrogen is -196 centigrade. When it comes into contact with the water in the pool (roughly room temperature, say 25 degrees) it will boil pretty fast, creating lots of gaseous nitrogen. The nitrogen gas is still very cold and therefore cools the air around it. With all the humidity around (humidity is a measure of how much water molecules are in the air), the water condenses into droplets, just as if we were high up in the sky where its pretty cold. This is why you see all those clouds in the picture.
Here's what I had the pleasure of doing with liquid nitrogen:
So far it seems pretty harmless. HOWEVER... if the amount of nitrogen is so large, than it will displace (push) the air around the pool, which means that the people in the pool will have less oxygen to breathe, and are likely to pass out/go into a comma/die/drown....you get the point. The bottom line is that the danger is asphyxiation and not poisoning (as suggested in the article)
The moral of the story is that never stay in the pool when liquid nitrogen is thrown in. Outside is better, and be sure that this is an open space.
What about the chlorine you're wondering? Its still in the water. Nothing bad happened to it. Thanks for asking.
I would like to see more newspapers talk to actual chemists before printing such comments within their published work in the future. Save us a lot of headache explaining why the journalists got it wrong.
Some people seems to think so.... and newspapers seem to publish this nonsense:
http://www.news.com.au/world-news/liquid-nitrogen-stunt-at-jagermeister-party-in-mexico-leaves-a-man-in-coma/story-fndir2ev-1226666959021
Lets get the fact right this time:
Nitrogen, in our case is the molecular form of nitrogen N-N or N2 (as opposed to the Nitrogen atom) is found in our atmosphere as gas, and accounts for about 78% (source: http://www.space.com/17683-earth-atmosphere.html). Thanks to technological innovations we can now make Liquid Nitrogen (http://blogs.howstuffworks.com/2009/07/27/how-do-they-make-liquid-nitrogen/)/
The boiling temperature of liquid nitrogen is -196 centigrade. When it comes into contact with the water in the pool (roughly room temperature, say 25 degrees) it will boil pretty fast, creating lots of gaseous nitrogen. The nitrogen gas is still very cold and therefore cools the air around it. With all the humidity around (humidity is a measure of how much water molecules are in the air), the water condenses into droplets, just as if we were high up in the sky where its pretty cold. This is why you see all those clouds in the picture.
Here's what I had the pleasure of doing with liquid nitrogen:
So far it seems pretty harmless. HOWEVER... if the amount of nitrogen is so large, than it will displace (push) the air around the pool, which means that the people in the pool will have less oxygen to breathe, and are likely to pass out/go into a comma/die/drown....you get the point. The bottom line is that the danger is asphyxiation and not poisoning (as suggested in the article)
The moral of the story is that never stay in the pool when liquid nitrogen is thrown in. Outside is better, and be sure that this is an open space.
What about the chlorine you're wondering? Its still in the water. Nothing bad happened to it. Thanks for asking.
I would like to see more newspapers talk to actual chemists before printing such comments within their published work in the future. Save us a lot of headache explaining why the journalists got it wrong.
Tuesday, 11 June 2013
Science to toy around with
I would like to introduce you to Slater Harrison from Pennsylvania in the US.
He's a science teacher, but even more so, he's a science lover.
How can I tell (since I have never met him in person)?
He has the most amazing website called:
Science Toy Maker
(http://www.sciencetoymaker.org/)
and what is the website all about? well.. its about science, but in a way which I relate on a personal level with my work with Pueblo Science. The website aims to do the following:
"All science toys and projects:
You see, Slater provides opportunity, not products. He provides knowledge, not withhold it. He provides tools for everyone who wishes to experience, enrich, experiment, and just want to have fun with the world we see around us.
And thanks to his work, I too had the opportunity to try my hands on flying one of his air surfers (the one called the "Spinny Bug"), together with my 6 years old daughter. Before I start the description, I can tell you that she had lots of fun making it and trying to fly it (although both of us need lots of practice).
SO here's what we did:
1. Start by cutting out the pattern
2. Tape the pattern on the 0.5mm thick foam (which you can get from Slater)
3. Cut along the middle line to get two gliders:
6. Fold each piece into half
7. Tape two folded pieces together, and you've got the glider 8. Let's go fly a glider 9. I got to try that too (I tried posting the video, but it didn't work. I'll try tomorrow)
Now I should point out that there are a lot more details and explanations on Slater's website, which is why I'm not taking the time repeating them here (there's enough redundancy on the internet already). So just go to http://www.sciencetoymaker.org/ and check it out, you won't regret it. What I can say is that the written explanations are accompanied by a video, narrated by Slater (with a lovely voice I must say). The video is done so well, that she could follow the instructions after just one viewing.
Thank you Slater for lots of great ideas, and I know I'll be enjoying more of them in the future.
He's a science teacher, but even more so, he's a science lover.
How can I tell (since I have never met him in person)?
He has the most amazing website called:
Science Toy Maker
(http://www.sciencetoymaker.org/)
and what is the website all about? well.. its about science, but in a way which I relate on a personal level with my work with Pueblo Science. The website aims to do the following:
"All science toys and projects:
- *are accessible (so cheap to make that nobody is excluded because of cost, and they don't require special skills, tools, materials, or work facilities beyond a kitchen).
- *have a "more about" page with explanations, historical context, related activities and high quality links for further research.
- *have clear step by step video directions or text instructions with lots of pictures."
You see, Slater provides opportunity, not products. He provides knowledge, not withhold it. He provides tools for everyone who wishes to experience, enrich, experiment, and just want to have fun with the world we see around us.
And thanks to his work, I too had the opportunity to try my hands on flying one of his air surfers (the one called the "Spinny Bug"), together with my 6 years old daughter. Before I start the description, I can tell you that she had lots of fun making it and trying to fly it (although both of us need lots of practice).
SO here's what we did:
1. Start by cutting out the pattern
2. Tape the pattern on the 0.5mm thick foam (which you can get from Slater)
4. Cut along the middle line again to get the two halves of the glider
5. Cut the extra bits on the ends (which will separate the pattern paper from the foam)6. Fold each piece into half
7. Tape two folded pieces together, and you've got the glider 8. Let's go fly a glider 9. I got to try that too (I tried posting the video, but it didn't work. I'll try tomorrow)
Now I should point out that there are a lot more details and explanations on Slater's website, which is why I'm not taking the time repeating them here (there's enough redundancy on the internet already). So just go to http://www.sciencetoymaker.org/ and check it out, you won't regret it. What I can say is that the written explanations are accompanied by a video, narrated by Slater (with a lovely voice I must say). The video is done so well, that she could follow the instructions after just one viewing.
Thank you Slater for lots of great ideas, and I know I'll be enjoying more of them in the future.
Friday, 7 June 2013
Chemicals Have Feelings Too!
I've recently wrote a blog post for my good friend Dorea, to post on her beautiful website
Chemicals Are Your Friends!
She got Mike Ellis to create amazing pictures to complement the text, and with skill and imagination, Mike has done superbly (I wish I could draw this good).
I won't copy and paste the blog here, cause you can just follow the link:
http://chemicalsareyourfriends.com/sliders/chemicals-have-feelings-too/
Enjoy
Chemicals Are Your Friends!
She got Mike Ellis to create amazing pictures to complement the text, and with skill and imagination, Mike has done superbly (I wish I could draw this good).
I won't copy and paste the blog here, cause you can just follow the link:
http://chemicalsareyourfriends.com/sliders/chemicals-have-feelings-too/
Enjoy
Thursday, 30 May 2013
Cow chemistry and toxic minds
It is far easier to put toxic thoughts in the general public mind than it is to put chemical at toxic level that would spread as far and persist as long.
Why?
Whenever there's a physically toxic effect due to a chemical compound in the population, an investigation is immediate. Regulations are verified, enforcement is increased, and the public safety is restored.
Whenever there's a mentally toxic effect due to propaganda against (a chemical for example), the general public tends to disregard previously attained scientifically knowledge, overlook numerous supporting facts as to the actual benign nature of the issue, and the fear and bias spreads and persist.
When we rely on "experts advice" how can we tell who is a genuine interest-free trust-worthy expert? It is hard. I faced such situations myself in the past, and am sure they will come again in the future.
But here's the decisive concept: numbers and relevance.
I already posted in the past on how it is easy to present a chemical as toxic, even though it may be a vitamin without which we surely will die. For the most part, amounts are a crucial piece of information we need to know in order to assess whether something is dangerous or not.
Jow Schwarcz said it clearly and elegantly just yesterday: http://blogs.nature.com/soapboxscience/2013/05/29/the-presence-of-a-chemical-is-not-the-same-as-presence-of-risk/
Why?
Whenever there's a physically toxic effect due to a chemical compound in the population, an investigation is immediate. Regulations are verified, enforcement is increased, and the public safety is restored.
Whenever there's a mentally toxic effect due to propaganda against (a chemical for example), the general public tends to disregard previously attained scientifically knowledge, overlook numerous supporting facts as to the actual benign nature of the issue, and the fear and bias spreads and persist.
When we rely on "experts advice" how can we tell who is a genuine interest-free trust-worthy expert? It is hard. I faced such situations myself in the past, and am sure they will come again in the future.
But here's the decisive concept: numbers and relevance.
I already posted in the past on how it is easy to present a chemical as toxic, even though it may be a vitamin without which we surely will die. For the most part, amounts are a crucial piece of information we need to know in order to assess whether something is dangerous or not.
Jow Schwarcz said it clearly and elegantly just yesterday: http://blogs.nature.com/soapboxscience/2013/05/29/the-presence-of-a-chemical-is-not-the-same-as-presence-of-risk/
I met Dr. Schwarcz once, at the University of Toronto back in 2011, when he gave a talk at the Chemistry Department entitled: "Are cows more trustworthy than chemists?"
(the reference to cow can be found here:
But putting cows aside, his talk was all about how people get wrong ideas about chemicals, their nature, their uses and how to interpret what they see/hear from others. An example was a chemical which is both in a cleaning product as well as in a frozen dinner.
Thinking back at Dr. Schwarcz talk, I can say he is part of why I am engaged today in presenting science to the general public, working hard to fight chemophobia, and just enjoying sharing my love of science with anyone who cares to listen. Thank you Dr. Schwarcz.
Labels:
chemicals,
chemist,
chemistry,
chemophobia,
cows,
Joe Schwarcz,
toxic
Monday, 13 May 2013
Look me in the eye and tell me this doesn't look like fun
My last post was a heads up for Science Rendezvous, an annual science festival held across Canada, which aims to "present a unified voice on the importance of science to society, and collectively generate a culture of discovery and innovation in Canada."
Bringing science to the people is, for me, an endeavor of great importance.
It shows people the complexity, the versatility, and most importance the omniprescence of science in everything we encounter in our lives.
Science and Technology enabled so many things that we take for granted:
Electricity (beofre, fire was the only way to produce light)
Refrigeration (before, you needed ice to cool stuff)
Engines (before, horses were used as the best means of transportation)
Medicine (before, mortality was so much higher)
Electronics (did people have blogs before computers were invented?)
Communications (when couriers and pigeons were the fastest way to share information over distances)
I'm just reading a book about the history of Science and Technology in the twentieth century, which brings so many exapmles of inventions and discoveries that have lead to great effects on human society (whether for better or worse we can argue on a different blog)
So shaking up scientists from their labs, getting them to face the public, stare them in the eyes and say "isn't this great?" is a reminder we all need.
Just take a look at all these great displays of science:
A water based organ:
A rover robot:
The Bernoulli Effect (source: http://uwaterloo.ca/institute-nanotechnology/community/outreach/science-rendezvous)
A giant colon you can walk through (source: http://www.marsdd.com/event/science-rendezvous/)
A fire tornado (source: http://www.uwindsor.ca/dailynews/2012-05-08/uwindsor-pair-to-promote-science-rendezvous-before-national-audience)
Can't wait to see what they come up with next year....
Bringing science to the people is, for me, an endeavor of great importance.
It shows people the complexity, the versatility, and most importance the omniprescence of science in everything we encounter in our lives.
Science and Technology enabled so many things that we take for granted:
Electricity (beofre, fire was the only way to produce light)
Refrigeration (before, you needed ice to cool stuff)
Engines (before, horses were used as the best means of transportation)
Medicine (before, mortality was so much higher)
Electronics (did people have blogs before computers were invented?)
Communications (when couriers and pigeons were the fastest way to share information over distances)
I'm just reading a book about the history of Science and Technology in the twentieth century, which brings so many exapmles of inventions and discoveries that have lead to great effects on human society (whether for better or worse we can argue on a different blog)
So shaking up scientists from their labs, getting them to face the public, stare them in the eyes and say "isn't this great?" is a reminder we all need.
Just take a look at all these great displays of science:
A water based organ:
A rover robot:
The Bernoulli Effect (source: http://uwaterloo.ca/institute-nanotechnology/community/outreach/science-rendezvous)
A giant colon you can walk through (source: http://www.marsdd.com/event/science-rendezvous/)
A fire tornado (source: http://www.uwindsor.ca/dailynews/2012-05-08/uwindsor-pair-to-promote-science-rendezvous-before-national-audience)
Can't wait to see what they come up with next year....
Friday, 10 May 2013
Its Science Carnival Season again in Canada - May 11 - Science Rendezvous
Tomorrow, May 11 2013, Canada will be celebrating Science, now in its 6th year.
The name of the game is SCIENCE RENDEZVOUS
Where? so many places across Canada, you're probably not too far from a location right now.
I'll save on words, because all the info is right here:
http://www.sciencerendezvous.ca/
I'll write some more after the event.
Hope you can make it. Spread the word. Its going to be GREAT!!!
The name of the game is SCIENCE RENDEZVOUS
Where? so many places across Canada, you're probably not too far from a location right now.
I'll save on words, because all the info is right here:
http://www.sciencerendezvous.ca/
I'll write some more after the event.
Hope you can make it. Spread the word. Its going to be GREAT!!!
Wednesday, 24 April 2013
To be or not to be ... a university student ?
It seems that there's a lot of heated debate going on about the state of university students in Canada, their (over)qualification and their (under)employment status.
If you read Yasmin Jaswal's account :
http://ca.finance.yahoo.com/blogs/insight/canada-grad-students-overeducated-underemployed-214613448.html
you may get the impression that young people should not go to university at all. Its a waste of time, since you're not likely to get a job.
BUT WAIT!!!!!
If you read what Statistics Canada has to say :
http://www.statcan.gc.ca/pub/81-599-x/81-599-x2009002-eng.htm
you see that university graduates are actually much better off than the rest of the labor market, since "the 2008 unemployment rates of individuals with university (4.1%) or college (4.9%) credentials with the unemployment rates of those with high-school graduation (6.6%) or less (12.0%)"
So what's going on? is a university degree (or a college one for that matter) is a good thing to have? or not?
The truth, it appears, lies between the numbers. More accurately, it seems that in the process of averaging everyone together, we are loosing the higher resolution of the situation. That is, the short answer is (as it usually the case) "it depends".
If you read through CIBC's report on the labor mismatch
http://research.cibcwm.com/economic_public/download/if_2012-1203.pdf
you see that while some professions are facing a labor surplus (meaning, less hiring, stagnating salaries), there are many professions which are experiencing a labor shortage.
With the risk of oversimplifying reality, I will conclude with this:
Q: To be or not to be ... a university student ?
A: It depends on what profession you are considering.
If you read Yasmin Jaswal's account :
http://ca.finance.yahoo.com/blogs/insight/canada-grad-students-overeducated-underemployed-214613448.html
you may get the impression that young people should not go to university at all. Its a waste of time, since you're not likely to get a job.
BUT WAIT!!!!!
If you read what Statistics Canada has to say :
http://www.statcan.gc.ca/pub/81-599-x/81-599-x2009002-eng.htm
you see that university graduates are actually much better off than the rest of the labor market, since "the 2008 unemployment rates of individuals with university (4.1%) or college (4.9%) credentials with the unemployment rates of those with high-school graduation (6.6%) or less (12.0%)"
So what's going on? is a university degree (or a college one for that matter) is a good thing to have? or not?
The truth, it appears, lies between the numbers. More accurately, it seems that in the process of averaging everyone together, we are loosing the higher resolution of the situation. That is, the short answer is (as it usually the case) "it depends".
If you read through CIBC's report on the labor mismatch
http://research.cibcwm.com/economic_public/download/if_2012-1203.pdf
you see that while some professions are facing a labor surplus (meaning, less hiring, stagnating salaries), there are many professions which are experiencing a labor shortage.
With the risk of oversimplifying reality, I will conclude with this:
Q: To be or not to be ... a university student ?
A: It depends on what profession you are considering.
Tuesday, 16 April 2013
Again! do it again!
Have you ever had an event that made you wonder about an idea which up to that point was so obvious you never stopped to think about its true meaning?
Well, it happened to me today.
I attended a great talk by Dr. Effie Sauer, who teaches at the University of Toronto Scarborough campus. She was telling us how they provide their undergraduate chemistry students with "real scientific research" experience. Which brings up the question (which were eloquently presented today as part of the talk) , what are the objectives of such a course? What are students suppose to learn from such a course?
The answer (of which I'm only bringing up only a small portion, with my apologies to Dr. Sauer) is :
In real science, things don't always work. In fact, they usually don't work the first time around (and mostly not even the second time around).
The problem with undergraduate chemistry teaching in universities (and correct me if your university is an exception) is that students are usually given experiments which work. They've been tested for years all across the globe, and everyone knows they work, which is great, since the students get a sense of accomplishment (if it works), and everyone is happy.
BUT....
Real science doesn't work like that. Actual research is about not knowing what you get, and more often than not, you don't even know why it didn't work.
And then it hit me - why do we call it "Re - Search" ?
Looking up the etymological background, I've found that the "Re" implies repetition, performing a tack intensively. The "Search" is the seeking part.
So what do we get ? Seeking! Again! and Again! and Again!
Why do we do it again? because it didn't work!!!!
And to think that I've been doing research for so many years, and not thinking about this obvious use of the term "Research"!
(I should point out that when I was doing my undergraduate degree in Chemistry, during the organic labs, some reactions did actually fail. At that we would confront our instructor and say "hey, this doesn't work!" as which we were told "yeh, it never did". My fellow students and I felt deceived. They knew it didn't work and made us do all this work for nothing. Peh. - shows you how little appreciation I had for failure at that stage)
Well, it happened to me today.
I attended a great talk by Dr. Effie Sauer, who teaches at the University of Toronto Scarborough campus. She was telling us how they provide their undergraduate chemistry students with "real scientific research" experience. Which brings up the question (which were eloquently presented today as part of the talk) , what are the objectives of such a course? What are students suppose to learn from such a course?
The answer (of which I'm only bringing up only a small portion, with my apologies to Dr. Sauer) is :
In real science, things don't always work. In fact, they usually don't work the first time around (and mostly not even the second time around).
The problem with undergraduate chemistry teaching in universities (and correct me if your university is an exception) is that students are usually given experiments which work. They've been tested for years all across the globe, and everyone knows they work, which is great, since the students get a sense of accomplishment (if it works), and everyone is happy.
BUT....
Real science doesn't work like that. Actual research is about not knowing what you get, and more often than not, you don't even know why it didn't work.
And then it hit me - why do we call it "Re - Search" ?
Looking up the etymological background, I've found that the "Re" implies repetition, performing a tack intensively. The "Search" is the seeking part.
So what do we get ? Seeking! Again! and Again! and Again!
Why do we do it again? because it didn't work!!!!
And to think that I've been doing research for so many years, and not thinking about this obvious use of the term "Research"!
(I should point out that when I was doing my undergraduate degree in Chemistry, during the organic labs, some reactions did actually fail. At that we would confront our instructor and say "hey, this doesn't work!" as which we were told "yeh, it never did". My fellow students and I felt deceived. They knew it didn't work and made us do all this work for nothing. Peh. - shows you how little appreciation I had for failure at that stage)
Monday, 8 April 2013
Earth shaking science
When people think chemistry, they think BOOM!!! cool explosions, loud noises. Ever wonder why is that?
Here's a great perspective from a chemist's view on the excessive use of explosions to 'sell' chemistry. Yes, science does sometimes have explosions, but for the most part, science exploration is not earth shaking in that sense.
http://philosophicallydisturbed.wordpress.com/2013/04/05/chemistry-explosions-are-all-bang-and-no-buck/
Working with children in the past few years, I have learned that there are many really cool and exciting experiments one can do with children, which are safe, yet entertaining, and yes, you can still learn a lot from them.
(Image source: http://en.wikipedia.org/wiki/Explosion)
Thursday, 4 April 2013
A chemical to reckon with indeed
I came across a classic April fools' hoax (thanks Dorea):
http://m.theatlanticwire.com/entertainment/2013/04/florida-djs-april-fools-water-joke/63798/
In a nut shell - 2 radio hosts of a local Florida country radio station told listeners that DIHYDROGEN MONOXIDE is in their water system.
Lets spell it out DI - HYDROGEN = 2 hydrogens, and MONO - OXIDE = 1 oxygen, so the chemical formula would be H2O. Looks familiar? Of course, this is plain old WATER.
Now, it is not my intent to ridicule the majority of the population who are not chemical-savvy, and would rightfully say : "if people expect 'water' and don't expect 'dihysrogen monoxide' they would naturally be caught off guard and would expect the worst. This is the whole point of media bullets - to alert people"
And this is why the prank works so well. Relying on human nature of habit - knowing that news bullets alert us of an issue of importance, but also, if we are used to a certain terminology, anything new will confuse us.
Just imagine how people would react if news will alert people of highly-luminescent radiation-emitting resistive device which can cause sever burning and damage your eye sight if looked directly at?
People would start wondering what it is and how can we avoid it! Would they understand it means 'light bulbs'? maybe, maybe not.
Back to the H2O story, though, it is interesting to note that this prank is by far not a new one. Apparently, it is more than 20 years old http://urbanlegends.about.com/library/bl_ban_dhmo.htm
And even more amazing is that there is a website dedicated to the idea, called http://dhmo.org/ listing all the bad stuff water can cause (all of which is true, by the way - for example, it is the main component of acid rain!)
The moral lesson of the story is that we should always exercise critical thinking about what we hear in the media, however scientific it may sound. I don't mean you should never believe anyone, since when we are unfamiliar with a field of study, we have to rely on the specialists for knowledge. But we should always remember that facts can be presented in many ways, leading you to different conclusions.
Science is about examining the world as objectively as possible (and believe me, there are many stories of scientists who failed to do just that, on which I will probably elaborate in the future...), but as soon as we take a few facts, and make a decision on how to present them (order, choice of words, etc.) they become subjective.
http://m.theatlanticwire.com/entertainment/2013/04/florida-djs-april-fools-water-joke/63798/
In a nut shell - 2 radio hosts of a local Florida country radio station told listeners that DIHYDROGEN MONOXIDE is in their water system.
Lets spell it out DI - HYDROGEN = 2 hydrogens, and MONO - OXIDE = 1 oxygen, so the chemical formula would be H2O. Looks familiar? Of course, this is plain old WATER.
Now, it is not my intent to ridicule the majority of the population who are not chemical-savvy, and would rightfully say : "if people expect 'water' and don't expect 'dihysrogen monoxide' they would naturally be caught off guard and would expect the worst. This is the whole point of media bullets - to alert people"
And this is why the prank works so well. Relying on human nature of habit - knowing that news bullets alert us of an issue of importance, but also, if we are used to a certain terminology, anything new will confuse us.
Just imagine how people would react if news will alert people of highly-luminescent radiation-emitting resistive device which can cause sever burning and damage your eye sight if looked directly at?
People would start wondering what it is and how can we avoid it! Would they understand it means 'light bulbs'? maybe, maybe not.
Back to the H2O story, though, it is interesting to note that this prank is by far not a new one. Apparently, it is more than 20 years old http://urbanlegends.about.com/library/bl_ban_dhmo.htm
And even more amazing is that there is a website dedicated to the idea, called http://dhmo.org/ listing all the bad stuff water can cause (all of which is true, by the way - for example, it is the main component of acid rain!)
The moral lesson of the story is that we should always exercise critical thinking about what we hear in the media, however scientific it may sound. I don't mean you should never believe anyone, since when we are unfamiliar with a field of study, we have to rely on the specialists for knowledge. But we should always remember that facts can be presented in many ways, leading you to different conclusions.
Science is about examining the world as objectively as possible (and believe me, there are many stories of scientists who failed to do just that, on which I will probably elaborate in the future...), but as soon as we take a few facts, and make a decision on how to present them (order, choice of words, etc.) they become subjective.
Labels:
aprils fools,
dihydrogen monoxide,
hoax,
prank,
toxic,
water
Wednesday, 27 March 2013
I have a friend. Her name is Oxygen.
I must start with an apology. I am not an apologetic guy, but I know when an apology is warranted (or so I would like to believe).
My last post was over a month ago, when I just came back from vacation. It took me this long to catch up on myself and find the right state of mind to write yet another post.
I hope you understand...
Yesterday I had a really amazing discussion with a good friend of mine, Dorea Reeser.
An altogether amazing person. She and a few friends had won the Scientific American Iron Egghead contest, making a video explaining adrenal glands in a funny and approachable fashion:
My last post was over a month ago, when I just came back from vacation. It took me this long to catch up on myself and find the right state of mind to write yet another post.
I hope you understand...
Yesterday I had a really amazing discussion with a good friend of mine, Dorea Reeser.
An altogether amazing person. She and a few friends had won the Scientific American Iron Egghead contest, making a video explaining adrenal glands in a funny and approachable fashion:
She then went on to start her own Facebook page - https://www.facebook.com/ChemicalsAreYourFriends which addresses the same issue I had raised on February 12 -
Which is all about how people think that "Chemicals" is "Bad", making a gross generalization of our reality (which I think some chemicals will be personally offended!)
The, Dorea got invited by Scientif American to write a bolg post for Scientific America, which was later posted on Salon.com .
Then she went on and started her own website http://chemicalsareyourfriends.com/
As you can see, a very active women.
So, Dorea, following your train of thoughts, I made friends with Oxygen. She is sometimes explosive, but I will suffocate without her. She makes fire burn wild, destroying life, but without her life could not have been there to start with. Such a dipolar personality, don't you think?
I'll make it short, so you can have time to read Dorea's words. Well worth reading...
Until next time...
Yours
Alon
Monday, 18 February 2013
Jet-lagged thoughts
I'm jet-lagged. Tired and sleepless.
All too common for flying abroad. And most people know already about Melatonin and how it helps regulate our sleep.
But it reminded me of some interesting research buzz happening lately
Treating Alzheimer's with Melatonin.
http://www.sciencedaily.com/releases/2012/09/120926110110.htm
http://www.bbc.co.uk/news/uk-scotland-glasgow-west-12879959
It still has a long way to go before science can prove/disprove the benefits of using Melatonin to combat the effects of Alzheimer's disease but initial studies seem promising.
(and at this point this is the extent of my sleep deprived rambling...)
All too common for flying abroad. And most people know already about Melatonin and how it helps regulate our sleep.
But it reminded me of some interesting research buzz happening lately
Treating Alzheimer's with Melatonin.
http://www.sciencedaily.com/releases/2012/09/120926110110.htm
http://www.bbc.co.uk/news/uk-scotland-glasgow-west-12879959
It still has a long way to go before science can prove/disprove the benefits of using Melatonin to combat the effects of Alzheimer's disease but initial studies seem promising.
(and at this point this is the extent of my sleep deprived rambling...)
Tuesday, 12 February 2013
A chemical is just a chemical after all
I just read a most inspiring article by Michelle M. Francl about chemophobia.
Yes, you read it right - CHEMOPHOBIA - the irrational fear of chemicals. It is this fear that makes people do anything from (a someone innocuous) switch from hard chemical cleaners to more environmentally friendly cleaning agents (which is not necessary a bad thing) to the more dangerous action of preventing life saving medication to your children.
But why do we fear chemicals?
Perhaps it is due to how media portrays them? (check out time 1:37 in Toxic Trespass)
Lets get something straight.
Chemicals can be bad for you! (and so do people)
Chemicals can also be good for you! (and so do people)
So how do we know which is good and which is bad? - Experience!
And the people who have experience with chemicals are called Chemists!
Here's an example.
A certain chemical has the following attributes:
When pure it comes as white to off-white powder
May be harmful if inhaled.
May cause respiratory tract irritation.
May be harmful if absorbed through skin.
May cause skin irritation.
May cause eye irritation.
May be harmful if swallowed.
The LD-50 (the lethal dose that would kill half of the rats tested) is only about 2 teaspoons per kg
Its chemical name is ascorbic acid
and here's the important point - you cannot function without it. This is Vitamin C.
The moral of the story is simple. Yes, chemicals can be bad for you. But it depends. How much of it is dangerous? in what form is it dangerous?
Chemicals should be treated as with anything else in life - in moderation and with care.
Don't accept things as dangerous just because they are "chemicals" (all matter around you is a chemical - even water), but also don't accept things as good for you just because they are "natural" or "organic" (they are still chemicals, like the extract of the bark of the Willow tree which is highly toxic, unlike its synthetically modified version of salicylate, better known as Aspirin, which has the same beneficial affects as the bark of the Willow tree, but much less toxic).
Yes, you read it right - CHEMOPHOBIA - the irrational fear of chemicals. It is this fear that makes people do anything from (a someone innocuous) switch from hard chemical cleaners to more environmentally friendly cleaning agents (which is not necessary a bad thing) to the more dangerous action of preventing life saving medication to your children.
But why do we fear chemicals?
Perhaps it is due to how media portrays them? (check out time 1:37 in Toxic Trespass)
Lets get something straight.
Chemicals can be bad for you! (and so do people)
Chemicals can also be good for you! (and so do people)
So how do we know which is good and which is bad? - Experience!
And the people who have experience with chemicals are called Chemists!
Here's an example.
A certain chemical has the following attributes:
When pure it comes as white to off-white powder
May be harmful if inhaled.
May cause respiratory tract irritation.
May be harmful if absorbed through skin.
May cause skin irritation.
May cause eye irritation.
May be harmful if swallowed.
The LD-50 (the lethal dose that would kill half of the rats tested) is only about 2 teaspoons per kg
Its chemical name is ascorbic acid
and here's the important point - you cannot function without it. This is Vitamin C.
The moral of the story is simple. Yes, chemicals can be bad for you. But it depends. How much of it is dangerous? in what form is it dangerous?
Chemicals should be treated as with anything else in life - in moderation and with care.
Don't accept things as dangerous just because they are "chemicals" (all matter around you is a chemical - even water), but also don't accept things as good for you just because they are "natural" or "organic" (they are still chemicals, like the extract of the bark of the Willow tree which is highly toxic, unlike its synthetically modified version of salicylate, better known as Aspirin, which has the same beneficial affects as the bark of the Willow tree, but much less toxic).
Friday, 8 February 2013
She's a witch, she's a witch! Burn her! Burn her!
I'm a Monty Python fan. Yes I am.
They're rough, unapologetic, and just plain mean. Which is how humor should be (sometimes...).
The reason I bring this up is due to a latest new report:
http://abcnews.go.com/International/wireStory/alleged-witch-burned-alive-papua-guinea-18434480
I doubt that the good people of Papua New Guinea took the time to check whether the accused women in fact weighs the same as a duck (for memory refresher, watch here http://youtu.be/zrzMhU_4m-g), but to be a bit more serious about this appalling incident, this is just one more example that shows the strength of education (or lack off). It is always easy to choose women as a source of all evil (as history has shown time and time again), but as people are more informed, they are provided with more barriers of logic (one would hope) to refute such non sensible accusations, and condemn a poor innocent women to such a fate.
May we never hear of such ghastly crimes.
They're rough, unapologetic, and just plain mean. Which is how humor should be (sometimes...).
The reason I bring this up is due to a latest new report:
http://abcnews.go.com/International/wireStory/alleged-witch-burned-alive-papua-guinea-18434480
I doubt that the good people of Papua New Guinea took the time to check whether the accused women in fact weighs the same as a duck (for memory refresher, watch here http://youtu.be/zrzMhU_4m-g), but to be a bit more serious about this appalling incident, this is just one more example that shows the strength of education (or lack off). It is always easy to choose women as a source of all evil (as history has shown time and time again), but as people are more informed, they are provided with more barriers of logic (one would hope) to refute such non sensible accusations, and condemn a poor innocent women to such a fate.
May we never hear of such ghastly crimes.
Monday, 4 February 2013
Can science explain how bees fly? and why does that matter?
I've recently watched a film with my daughter. You've probably seen it, and most definitely have heard of it. Its called "The Bee Movie".
It starts with a statement:
It starts with a statement:
"According to all known laws of aviation, there is no way a bee should be able to fly.
Its wings are too small to get its fat little body off the ground.
The bee, of course, flies anyway because bees don't care what humans think is impossible."

Now, the statement in itself may seem amusing, since it tries to convey the fact that
nature doesn't need our permission to do what it has been doing since ever,
BUT....
the flaw in the argument is a very important point, which is
Science does not assume nature need to conform to its authority
Science merely exists to explain how nature bahves
It is the notion of divinity that assumes nature was given to humans to be ruled.
The biggest problem with having such joked in major pop films is that they help propagate
a false impression of what science is.
(just as a side note, looking up online whether science can explain bee's flight brings up some
neat anecdotes but are not my intention to include in this post)
Subscribe to:
Posts (Atom)




























