It is merely a table. Or is it.... I first heard Tom Lehrer sing "The Elements" song when I was taking my first year general chemistry course more than a decade ago.
"These are the only ones of which the news has come to Harvard,
And there may be many others, but they haven't been discahvahd"
And here lies the true wonder of the graphical masterpiece commonly known as "The Periodic Table of the Elements". Its name is misleading, since by the use of the word 'table' one may expect nothing more than "an orderly arrangement of data". But the periodic table is actually something completely different.
It is ... (wait for it, building the suspense here) .... A GRAPH!!!!
Or more precisely, an amalgamation of many graphs!!!
Yes, that's the truth.
What is the difference you may ask?
Well, a table is usually a way of presenting information in a tidy fashion to make it easier to find specific information of relevance. But a graph is a much more powerful tool. A graph plots values that are correlated to two or more attributes. Once plotted, trends can sometimes be observed. And if a trend exists - you can PREDICT! After all, science is more than just observing nature and taking notes. Science is about using the earlier observations in order to predict the outcome of future experiments (aka forming hypotheses)!
When Dimitri Ivanovich Mendeleev first published his periodic table of the elements in 1869, the elements were (for the most part) arranged based on their molecular weights. Mendeleev noticed that when arranging the elements according to their molecular weights (since atomic numbers were not yet a measurable quantity at that time - see footnote), you can arrange the elements in such a way that certain periodicities arise with respect to the properties of the elements. But the true breakthrough in Mendeleev's approach was that he then utilized his discovered pattern to predict new elements which had not yet been discovered. By using the periodic trends in the properties of the elements, he was able to predict some of the properties of those yet-to-be-discovered elements. And guess what ... he was right. Shortly after, Gallium, Scandium and Germanium were discovered, corroborating Mendeleev's hypothesis and exemplifying the practicality of the periodic table of the elements.
I mentioned the word "periodicity" several times, but periodicity of what? The answers is: quite a fair bit. Let's look at how the Ionization energy of the various elements changes when ordered in the periodic table arrangement. (The ionization energy is the amount of energy needed to separate one electron from the initially neutral atom).
 In the above graph, the height of each element corresponds to its first ionization energy (in eV units).
So what can we tell by looking at the graph? The first obvious observation is that when moving down each column, the height decreases (granted there are some exceptions, but let's look at the general rule). Another observation is that when moving from left to right along each row, the height generally increases, although several sharp drops are seen (such as in the case of N-nitrogen and O-oxygen or Cd-cadmium and In-indium).
You can check out some more examples at www.chemicool.com .
Atomic radius, ionization energy, melting point, boiling point, density; they all show a periodic behavior when plotted against their atomic numbers (again see footnote).
Why do we get such periodicities you might be wondering?
The atomic number, the number of protons in the nucleus of the element, provides the basis of the periodicities in the periodic table. The more protons there are , the heavier the atom is. Additionally, the atomic number also indicates the number of electrons around the nucleus (since the atoms in their pure state are neutral, therefore for every positively charged proton in the nucleus there will be a negatively charged electron around it). As the number of electrons increases, the atomic radius becomes larger (with exception of 'kinks' due to periodic changes in the arrangement of the electrons, similar to the sudden sharp drops we saw for the ionization energies). And since electrons are the main players in chemical reactions, the number of electrons and their specific arrangement around the nucleus will affect the reactivity of the element.
In chemistry, electrons like to be paired. Just like people (nudge, nudge, wink, wink). Let's take a look at the group with a common attribute along a vertical line in the periodic table: Lithium, Sodium and Potassium. They all have a single unpaired electron, and are similarly reactive because an unpaired electron is more reactive being all by itself.
In the above graph, the height of each element corresponds to its first ionization energy (in eV units).
So what can we tell by looking at the graph? The first obvious observation is that when moving down each column, the height decreases (granted there are some exceptions, but let's look at the general rule). Another observation is that when moving from left to right along each row, the height generally increases, although several sharp drops are seen (such as in the case of N-nitrogen and O-oxygen or Cd-cadmium and In-indium).
You can check out some more examples at www.chemicool.com .
Atomic radius, ionization energy, melting point, boiling point, density; they all show a periodic behavior when plotted against their atomic numbers (again see footnote).
Why do we get such periodicities you might be wondering?
The atomic number, the number of protons in the nucleus of the element, provides the basis of the periodicities in the periodic table. The more protons there are , the heavier the atom is. Additionally, the atomic number also indicates the number of electrons around the nucleus (since the atoms in their pure state are neutral, therefore for every positively charged proton in the nucleus there will be a negatively charged electron around it). As the number of electrons increases, the atomic radius becomes larger (with exception of 'kinks' due to periodic changes in the arrangement of the electrons, similar to the sudden sharp drops we saw for the ionization energies). And since electrons are the main players in chemical reactions, the number of electrons and their specific arrangement around the nucleus will affect the reactivity of the element.
In chemistry, electrons like to be paired. Just like people (nudge, nudge, wink, wink). Let's take a look at the group with a common attribute along a vertical line in the periodic table: Lithium, Sodium and Potassium. They all have a single unpaired electron, and are similarly reactive because an unpaired electron is more reactive being all by itself.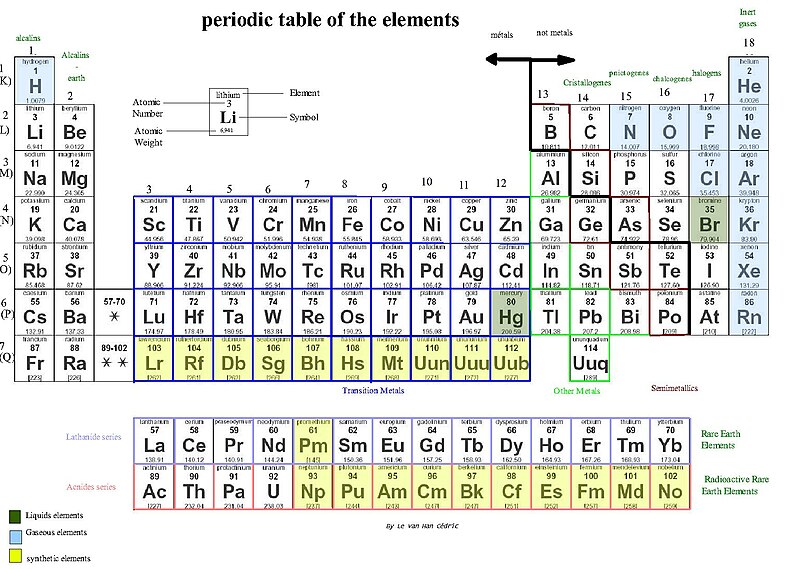 Contrary to the above group of elements, Helium, Neon and Argon have all their electrons paired. They are all similarly nonreactive and belong to the same group along a vertical line in the periodic table.
(In fact, the electronic structure is more complicated than simple pairing, which is why we saw fluctuations in the above graph where the ionization energy showed sharp drops in the general increasing trend moving from left to right along the rows. Since this post is getting quite long, I'll leave such descriptions to another time)
The periodic 'table' contains a plethora of information. Graphically, it is (probably) the most concise form to summarize an astounding amount of information.
Contrary to the above group of elements, Helium, Neon and Argon have all their electrons paired. They are all similarly nonreactive and belong to the same group along a vertical line in the periodic table.
(In fact, the electronic structure is more complicated than simple pairing, which is why we saw fluctuations in the above graph where the ionization energy showed sharp drops in the general increasing trend moving from left to right along the rows. Since this post is getting quite long, I'll leave such descriptions to another time)
The periodic 'table' contains a plethora of information. Graphically, it is (probably) the most concise form to summarize an astounding amount of information.So next time you gaze at the periodic table, remember, it is more than 'just' a table. It is the essence of the chemistry that makes up our entire universe!
Footnote: The numbers we see today as the basic ordering of the elements are the Atomic Numbers. These are the number of protons in the nucleus of each of the elements, but they were only discovered in 1913 by Henry Moseley, which makes Mendeleev's accomplishment even more impressive.

No comments:
Post a Comment